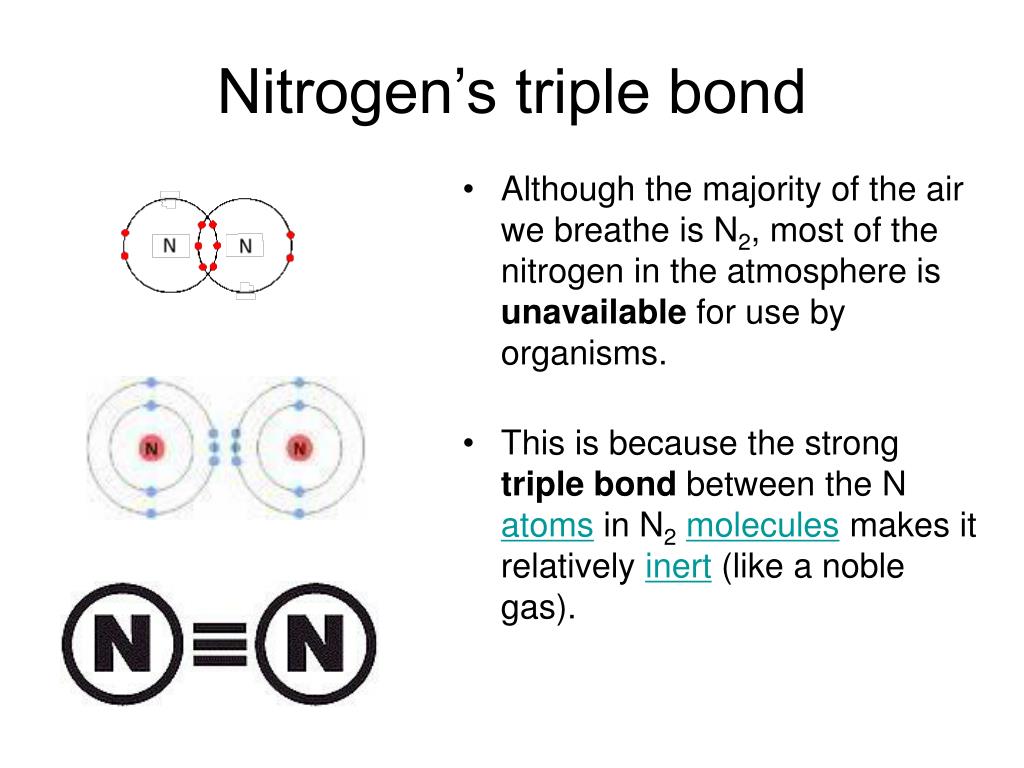
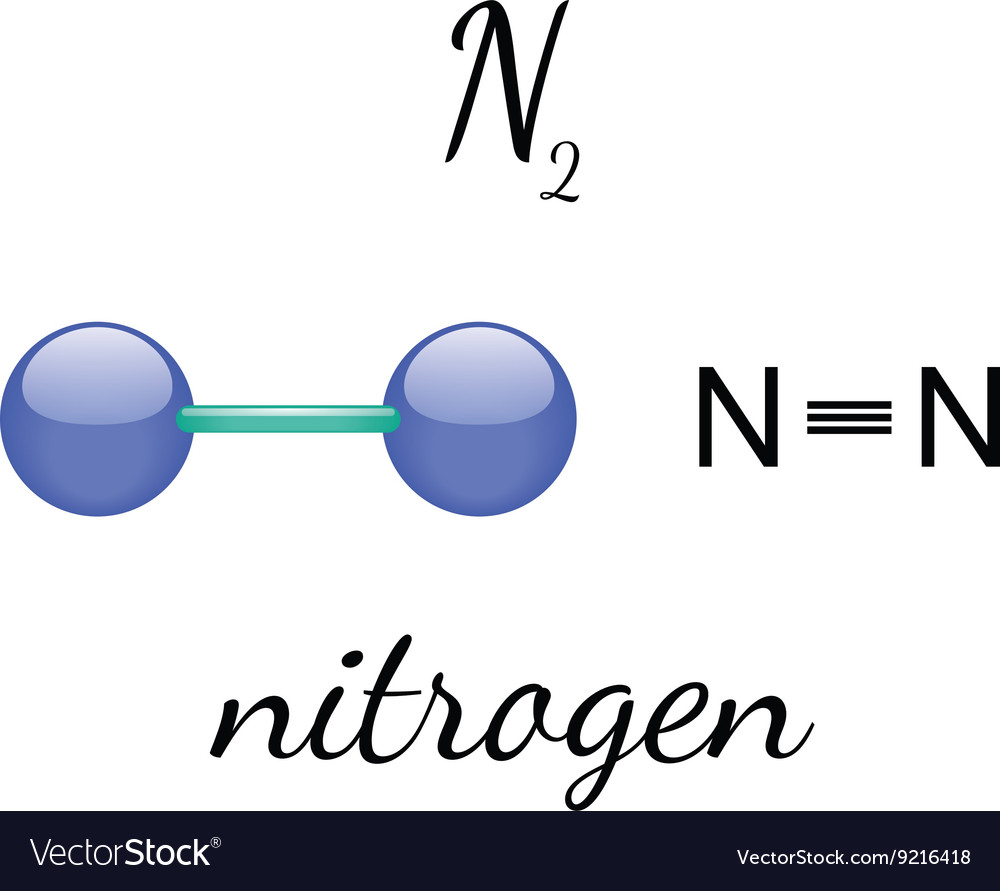
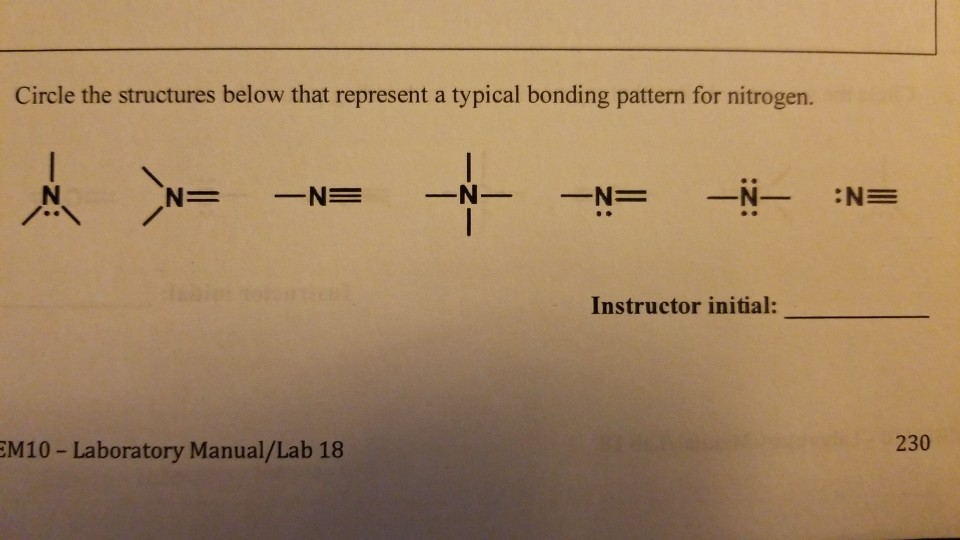
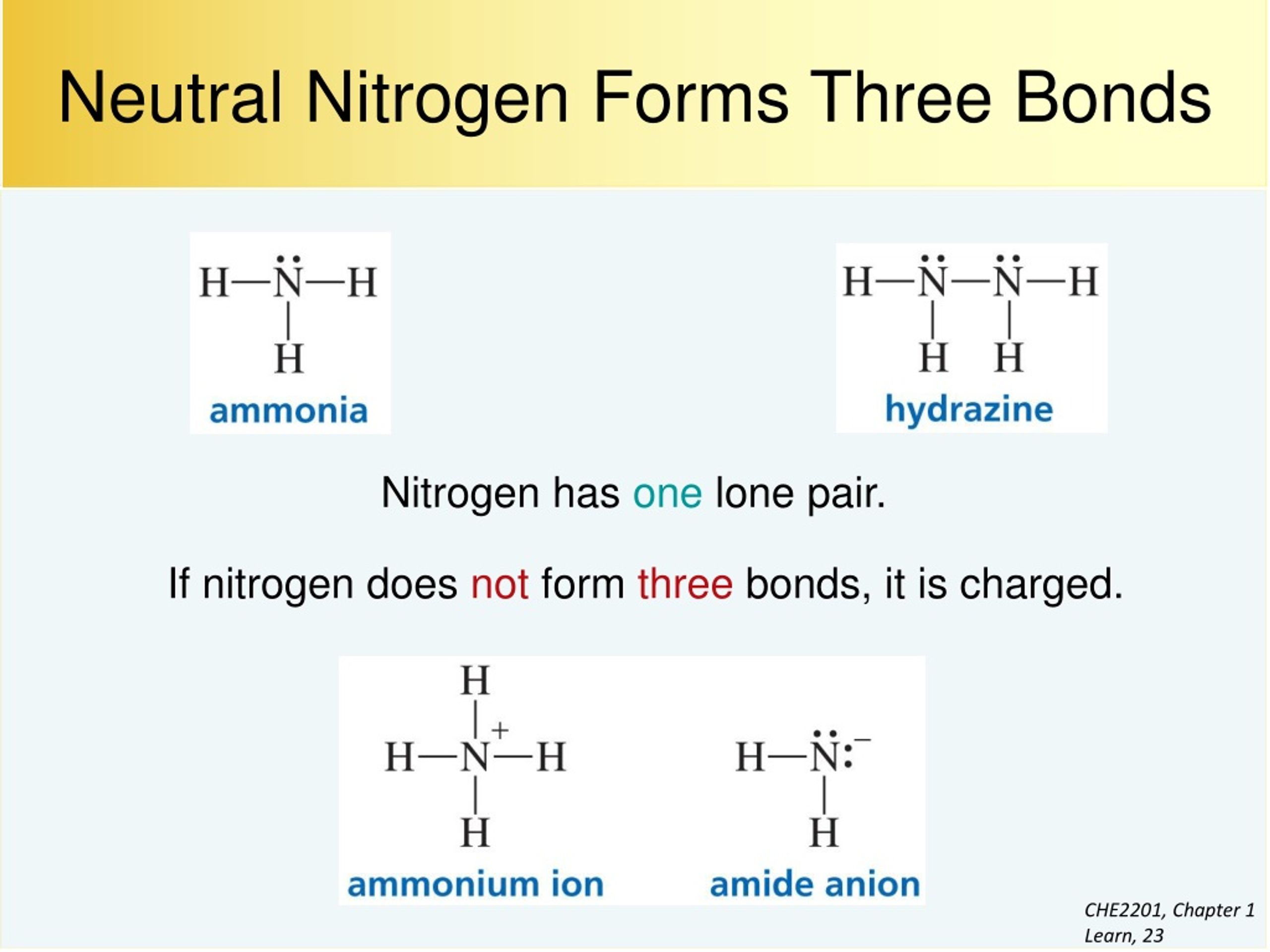
Sharing Electrons. A covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. The shared electrons are attracted to the nuclei of both atoms. This forms a molecule consisting of two or more atoms. Covalent bonds form only between atoms of nonmetals.. The most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share electrons. Nitrogen Forms 3 Bonds. This type of bond is very strong, which makes it useful in applications like explosives. Nitrogen can also form a double bond, in which two nitrogen atoms share electrons. This type of bond is not as strong as the.

The Valency Of Nitrogen In Nh3 The Valency Of Nitroge vrogue.co

How Many Bonds Does Nitrogen Form Asking List
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID899587
How Many Bonds Does Nitrogen Form Asking List
N2 nitrogen molecule Royalty Free Vector Image

Covalent Compound Examples
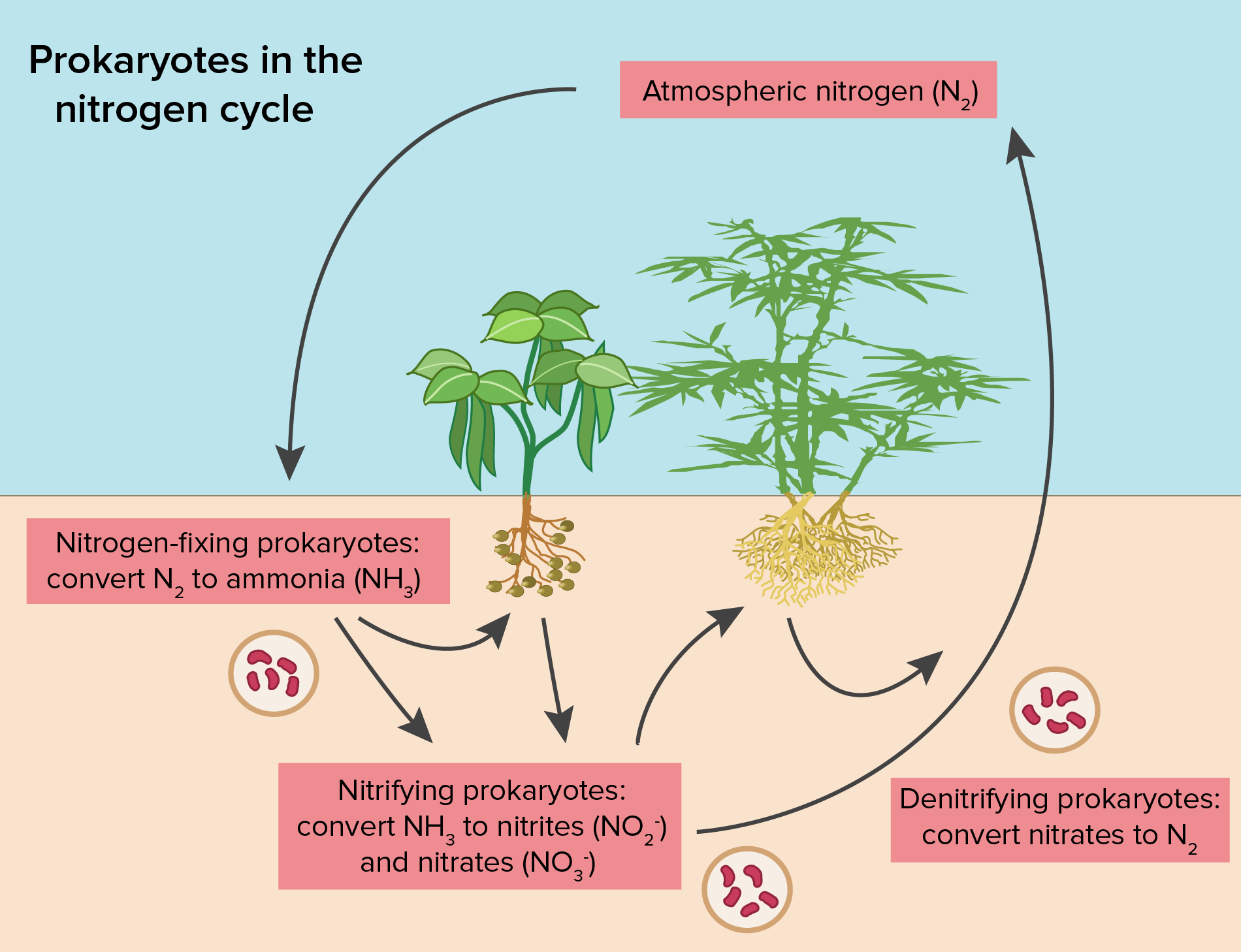
Describe How Fixed Nitrogen Is Used by Plants RaulhasKane
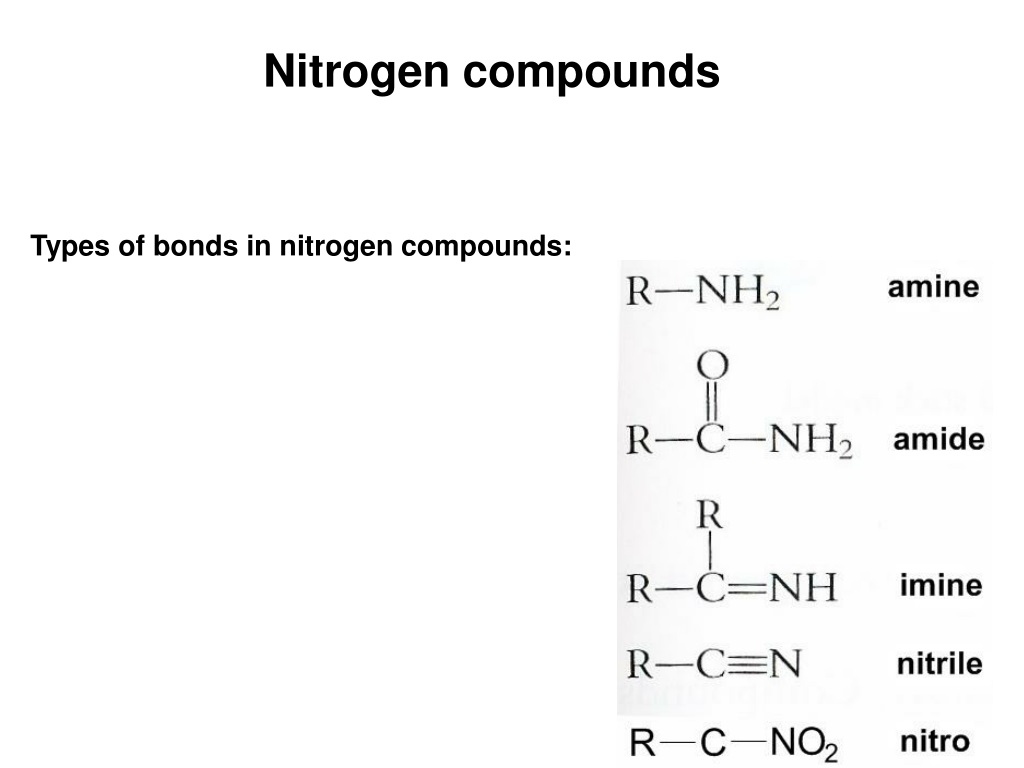
Grade 12 CHAPTER 4 NITROGEN COMPOUNDS. SEMESTER 1

How many bonds does Nitrogen form? YouTube
Specific Heat Examples Everyday Life
How Many Bonds Does Nitrogen Form Asking List

__TOP__ How Many Covalent Bonds Can Chlorine Form
How Many Bonds Does Nitrogen Form Asking List

Covalent Bond Definition Types Properties And Example vrogue.co
How Many Bonds Does Nitrogen Form Asking List
image

How to draw correlation diagrams chemistry talentper

Bond formation in nitrogen molecule Stock Image C028/6485 Science Photo Library
how many bonds does sulfur form

Why Nitrogen can't form 5 bonds or Pentahalides ? Class 11 NEET GURU YouTube
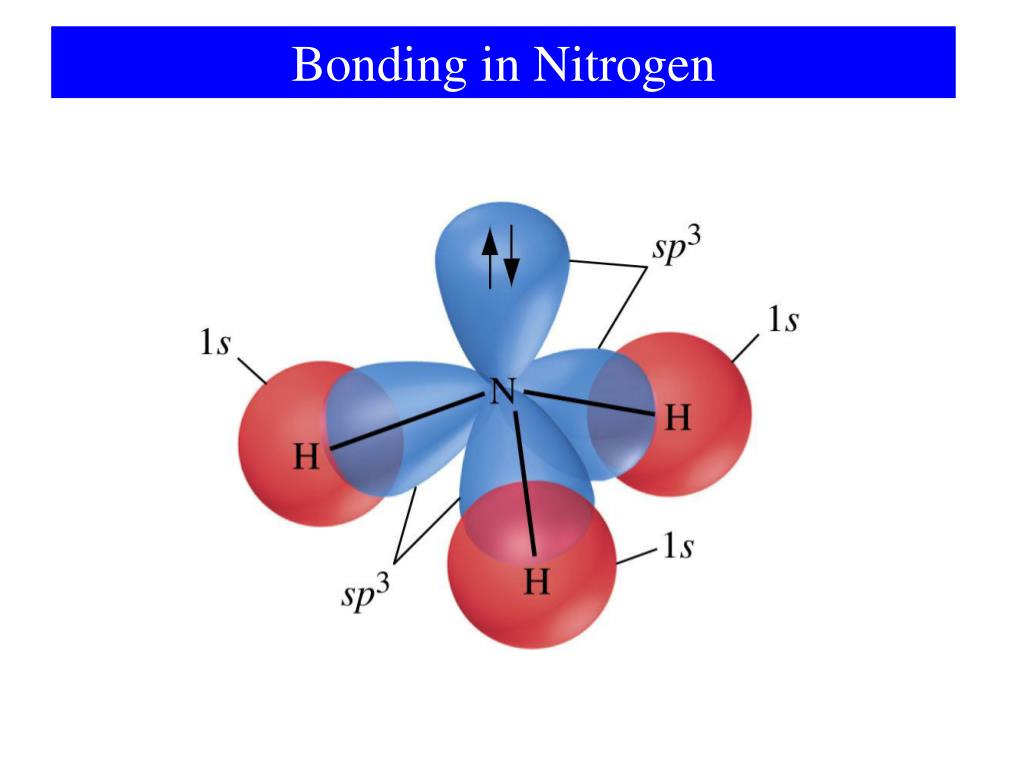
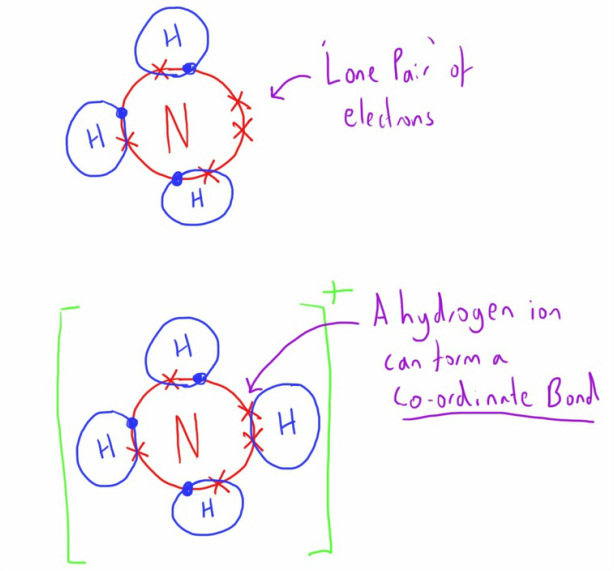
Nitrogen has one lone pair of electrons in its 2s orbital. It can donate this electron pair to form a coordinate bond. This coordinate bond that nitrogen forms by donating its electron pair to the vacant orbital of other atom is how it can form 4 bonds. Share. Cite.. Bonding of Oxygen and Fluorine. Let us now consider oxygen (O) which has eight electrons, two in the core and six valence (1s2, 2s2, 2px1, 2py1, 2pz1). As with nitrogen, oxygen does not use all its electrons to form six bonds because it is too small and the orbitals that would need to be used to make six bonds are too high in energy to be.